FXR agonists for NASH: How are they different and what difference do they make?
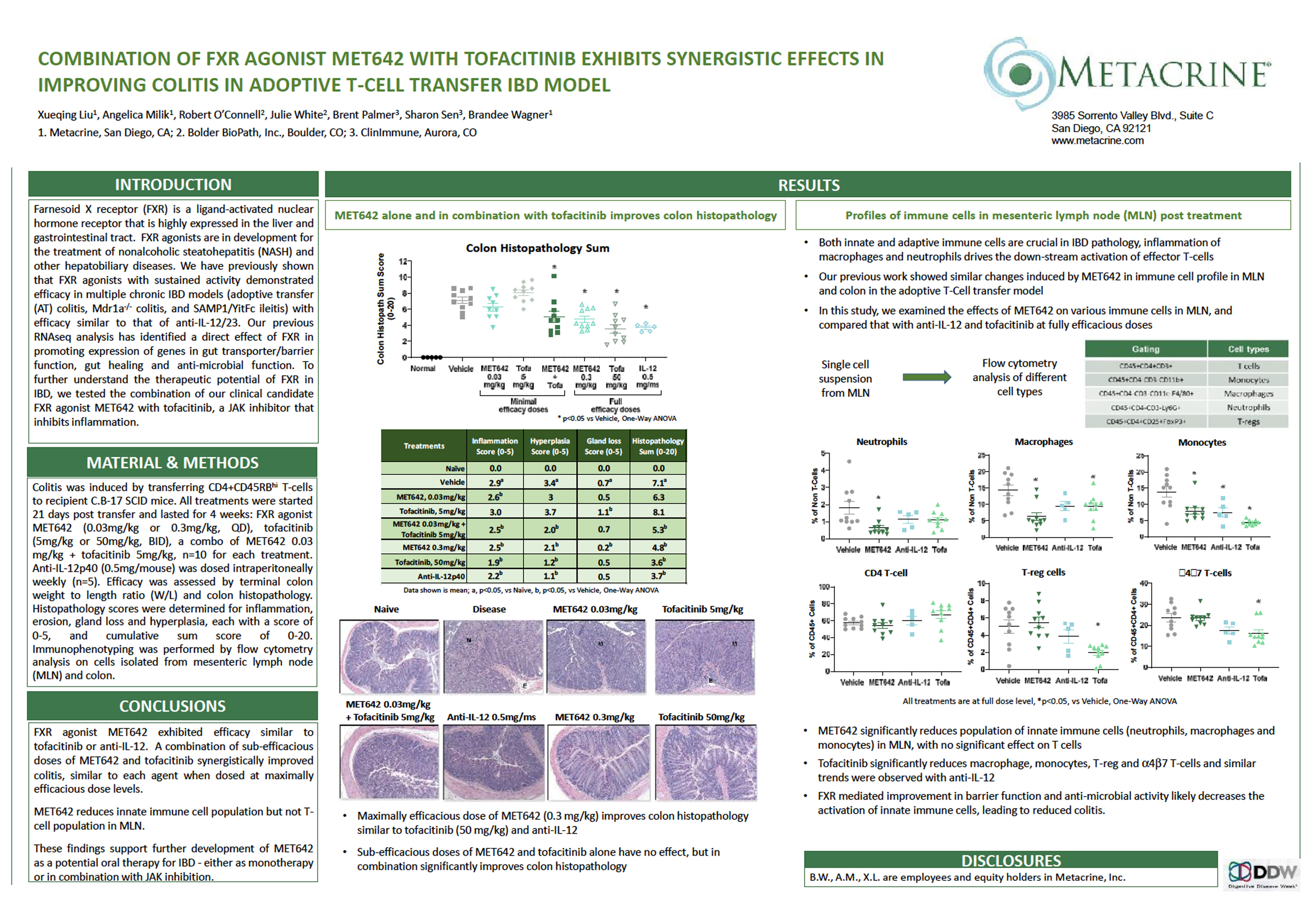
Combination of FXR Agonist MET642 With Tofacitinib Exhibits Synergistic Effects in Improving Colitis in Adoptive T-Cell Transfer IBD Model
A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis
Stephen A.Harrison1 2, Mustafa R.Bashir3, Kyoung-JinLee4, JenniferShim-Lopez4, JonathanLee4, BrandeeWagner4, Nicholas D.Smith4, Hubert C.Chen4, Eric J.Lawitz56
1. Summit Clinical Research, San Antonio, TX, USA
2. Pinnacle Clinical Research, San Antonio, TX, USA
3. Duke University Medical Center, Durham, NC, USA
4. Metacrine, Inc., San Diego, CA, USA
5. Texas Liver Institute, San Antonio, TX, USA
6. University of Texas Health San Antonio, San Antonio, TX, USA
Received 19 October 2020, Revised 28 January 2021, Accepted 29 January 2021, Available online 11 February 2021.
Highlights
- The benefit of FXR agonism in patients with NASH has been clinically validated.
- However, improvements in efficacy/tolerability for the therapeutic class have been elusive.
- MET409, a novel non-bile acid agonist, resulted in a class-leading 12-week relative liver fat reduction of 38–55%.
- MET409 resulted in differentiated pruritus and LDL-cholesterol profiles – issues that have hampered FXR development.
- Data provide first evidence that the risk-benefit profile of FXR agonists can be enhanced through structural optimization.
Background & Aims
The benefits of farnesoid X receptor (FXR) agonists in patients with non-alcoholic steatohepatitis (NASH) have been validated, although improvements in efficacy and/or tolerability remain elusive. Herein, we aimed to assess the performance of a structurally optimized FXR agonist in patients with NASH.
Methods
In this 12-week, randomized, placebo-controlled study, we evaluated MET409 – a non-bile acid agonist with a unique chemical scaffold – in patients with NASH. Patients were randomized to receive either 80 mg (n = 20) or 50 mg (n = 19) of MET409, or placebo (n = 19).
Results
At Week 12, MET409 lowered liver fat content (LFC), with mean relative reductions of 55% (80 mg) and 38% (50 mg) vs. 6% in placebo (p <0.001). MET409 achieved ≥30% relative LFC reduction in 93% (80 mg) and 75% (50 mg) of patients vs. 11% in placebo (p <0.001) and normalized LFC (≤5%) in 29% (80 mg) and 31% (50 mg) of patients vs. 0% in placebo (p <0.05). An increase in alanine aminotransferase (ALT) was observed with MET409, confounding Week 12 changes from baseline (−25% for 80 mg, 28% for 50 mg). Nonetheless, MET409 achieved ≥30% relative ALT reduction in 50% (80 mg) and 31% (50 mg) of patients vs. 17% in placebo. MET409 was associated with on-target high-density lipoprotein cholesterol decreases (mean changes of −23.4% for 80 mg and −20.3% for 50 mg vs. 2.6% in placebo) and low-density lipoprotein cholesterol (LDL-C) increases (mean changes of 23.7% for 80 mg and 6.8% for 50 mg vs. −1.5% in placebo). Pruritus (mild-moderate) occurred in 16% (50 mg) and 40% (80 mg) of MET409-treated patients.
Conclusion
MET409 lowered LFC over 12 weeks in patients with NASH and delivered a differentiated pruritus and LDL-C profile at 50 mg, providing the first clinical evidence that the risk-benefit profile of FXR agonists can be enhanced through structural optimization.
Lay summary
Activation of the farnesoid X receptor (FXR) is a clinically validated approach for treating non-alcoholic steatohepatitis (NASH), although side effects such as itching or increases in low-density lipoprotein cholesterol are frequently dose-limiting. MET409, an FXR agonist with a unique chemical structure, led to significant liver fat reduction and delivered a favorable side effect profile after 12 weeks of treatment in patients with NASH. These results provide the first clinical evidence that the risk-benefit profile of FXR agonists can be enhanced.
Graphical abstract